Balloon procedures in ENT can be highly variable between operators and difficult to standardize outside the operating room.
Navigation can be limited
Anatomy varies widely
Real-time visualization is often insufficient
This makes office-based procedures harder than they should be—reducing efficiency, consistency, and confidence.
ExperiENT is a single platform, built for real-world in-office ENT workflows, that brings together:

Visualization

Balloon technology

Procedural intelligence
The goal is simple: help ENT physicians perform office procedures with greater precision, fewer steps, and more predictable outcomes.
ExperiENT combines visualization, balloon technology, and procedural intelligence into one unified platform.
How it works

Camera-guided access provides real-time orientation
Integrated balloon + visualization reduces guesswork

Software assists with workflow, consistency, and decision-support

Designed specifically for office-based procedures— not adapted OR tools
Why It Matters

More confidence in challenging anatomy

More standardized experiences between operators

Improved patient comfort and faster in-office workflow

A scalable platform for future ENT technologies and indications
30M+ U.S. adults diagnosed withsinusitis annually
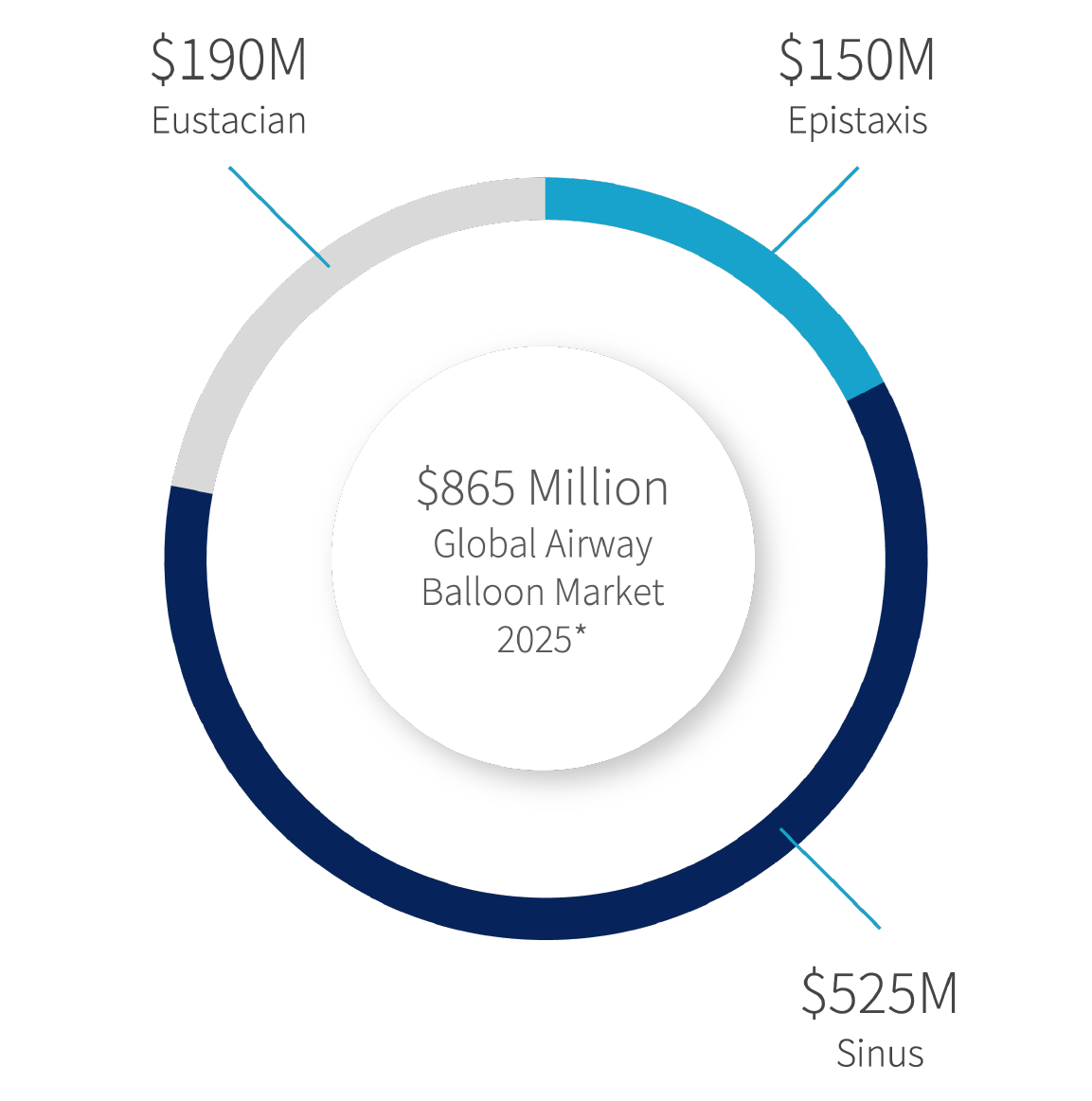
Eustachian
Sinus
Epistaxis







